We have been often asked how the on-site results of the 3AWater Multimetal Water Analysis System (MWAS) compare with standard laboratory analysis. Since comparing measurements and analysis techniques is quite complex theme, we figured to write a short blog post about measurement errors and how they affect laboratory and on-site analysis methods. This blog post is written from the perspective of dissolved metal analysis of environmental waters, but the discussion can also be applied to other analyses.
Errors come in many different forms
The typical errors sources in laboratory analysis including conventional sampling are following:
Sampling error is related on taking the sample to be delivered to the laboratory for the analysis. This includes human errors of improper sampling such as contamination of the sample, and also any differences between the taken sample and the average of the entire body of water being samples. Typical situation in water analysis is that water source is not homogenous, but there are water quality differences depending on, for example, on the depth the sample is taken from a lake. Sampling error is laborious to quantify and often ignored even though it may have a significant contribution on the total error. However, in systematic studies sampling errors as high as 40% have been reported.
Transportation and storage error is related to events that take place between the sampling and the measurement in the lab. There can be chemical, physical or biological interactions that change the chemistry and composition of a sample during transportation and storage. In case of dissolved metal analysis of waters, low metal concentrations can often precipitate on the surfaces of the containers used in sampling affecting to the laboratory analysis. Another unfortunately common source of error is leaching of metals from containers especially into acidic waters.
Systematic error is related to the analysis method itself and how close the analysis result is to the “true value”. There can be a bias shifting the measured values systemically from the true value because of water matrix (other components in the water than the one being measured) is affecting the measurement. There can also be systematic errors because of improper calibration, instrument malfunction or user error. The methods ability to hit the “true value” is called the accuracy.
Random errors are observed as a variance between repeated measurements of the same sample. These errors are random in nature, i.e., they are dispersed around their average and are due to the operator or the technical limitations of the method and technology itself. Commercial laboratories typically promise 10 – 15 % error for dissolved metal analysis of waters.
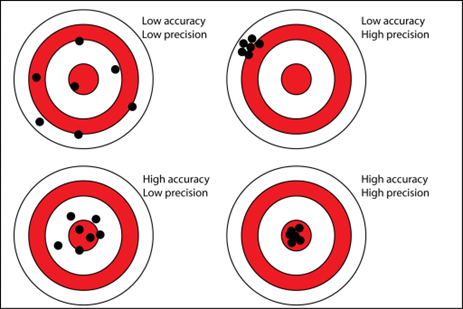
Quality work to control the errors
All these errors are included in a total error of a typical laboratory analysis, and they can have a significant effect on the results. There are several ways to control these errors with quality control.
Proper sampling and sample preparation is extremely important, since this can create huge errors and even make the whole analysis useless. In environmental water analysis, sample is typically taken from flowing water to get a representative sample and sampling equipment is washed three times with the same water in order to exclude cross contamination from the previous samples. Samples for dissolved metal analysis are filtrated and preserved with an acid either on field or send to laboratory for filtration and acidification as soon as possible to prevent precipitation of dissolved metals or dissolution of precipitated metals. Environmental water samples should also be stored in cooler bags during transport and in refrigerators for storage. Preparing a so called zero sample is a good way to check if contaminations are introduced into the samples during sampling and storage. This means preparing and storing a pure water sample in a similar way as the environmental samples to see if any contaminants will be found in the zero sample.
Frequent calibrations of the analytical devices and comparison of the results with other analytical methods or samples with known composition is important in order to keep the systematic errors in control. The commercial laboratories in Finland, for example, are calibrating their instruments daily and participating on laboratory proficiency tests (see web pages of The Finnish Environment Institute for more information).
Making several parallel measurements from the same sample is effective way to control random error, since averaging the analysis results should reduce the random error.
Taking several samples from the same point is an effective way to get reliable analysis and to investigate the errors involved in the whole process. The average will give a more representative result and deviation between the results gives information on the reliability of the measurements. However, this increases the costs of the analysis quite much and in case of dissolved metal analysis of waters it is typical to take only one sample per point for the analysis.
On-Site Methods vs. Laboratories
Besides laboratory methods, the different on-site analysis technologies have been developed for faster operations in field conditions. The on-site methods can be especially useful when results are needed for fast decision making on-site. The applications of fast data-based decision making in an industrial setup can be:
- Operating and optimizing water treatment processes to make sure output water is within the quality limits.
- Ensure the cleanliness of discharge water.
- Pin-pointing leakages and sources of contaminations.
- Smart sampling: Deciding the sampling points based on the results of the earlier samples to focus the analysis on relevant areas and to get more relevant data.
An advantage of the on-site analysis methods is also that errors related to the transport and storage are excluded. The fast results allow taking another sample and making another analysis if abnormal results is observed to confirm the results.
Laboratory instruments on, the other hand, offer typically better accuracy and precision, lower detection limits and broader list of analysed parameters than on-site methods. Accredited laboratory services fulfil the requirements for monitoring for authority and permit purposes, which is often important when choosing analysis service and methods. However, the devices are typically expensive and need highly trained personnel and a lot of regular maintenance.
On-site technologies and laboratory instruments or services have quite different purpose. When the data quality of on-site measurements if sufficient they are cost-efficient ways of getting data quickly for decision making. The purpose of the laboratory instruments on the other hand is to give ultimate performance. Because of these differences, direct comparison of the features or performance between these different types of analyses is typically not very meaningful.


